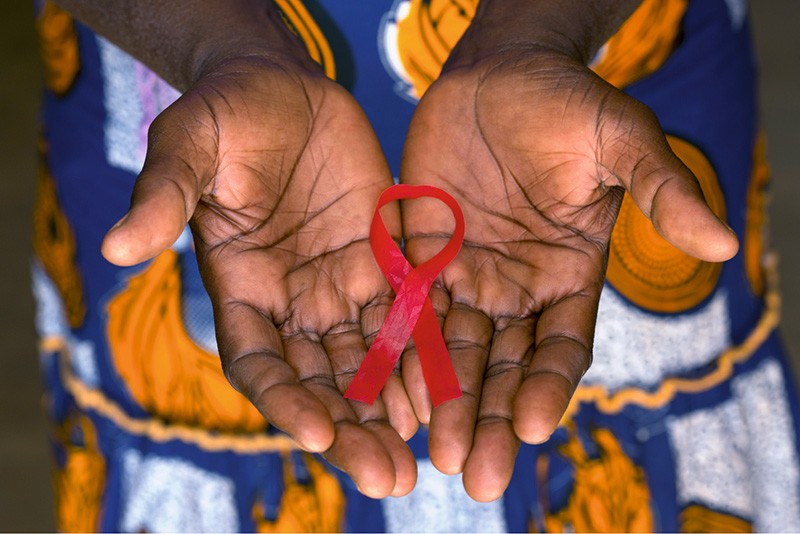
Gilead Sciences, a US-based biopharma company, has announced a major step towards expanding access to HIV prevention. The company has partnered with six pharmaceutical manufacturers to produce generic versions of its HIV prevention drug, lenacapavir, in 120 low- and lower-middle-income countries, including Nigeria and Kenya.
Lenacapavir, administered as a twice-yearly injection, has been proven highly effective in preventing HIV infection.
The drug will soon be made available in 120 high-incidence, resource-limited regions, offering new hope in the global fight against a virus that has claimed more than 25 million lives over the past two decades.
In a statement on its website, Gilead said it will first prioritise 18 countries for the drug rollout. The 18 countries, according to the company, “represent about 70% of the HIV burden in the countries named in the license.” These nations were identified through consultations with external partners and include Botswana, Eswatini, Ethiopia, Kenya, Lesotho, Malawi, Mozambique, Namibia, Nigeria, Philippines, Rwanda, South Africa, Tanzania, Thailand, Uganda, Vietnam, Zambia, and Zimbabwe.”
Clinical trials for lenacapavir have shown promising results, particularly among at-risk populations. In one trial involving girls and women in South Africa and Uganda, the drug successfully prevented HIV infection. A separate trial involving men in Argentina, Brazil, Mexico, Peru, South Africa, Thailand, and the US demonstrated near-complete protection from the virus.
However, despite its success, lenacapavir is currently priced at 68 million naira ($42,250) per year in the United States, where it is sold under the brand name Sunlenca. Researchers estimate that the drug could be produced for as little as 64 thousand naira ($40) per patient annually, making it far more accessible in lower-income regions.
It is to bo bridge this affordability gap, Gilead signed agreements with six manufacturers to produce and distribute generic versions of lenacapavir at significantly lower costs in countries where HIV incidence is highest. This initiative is expected to improve access to HIV prevention in resource-limited countries, where millions remain vulnerable due to high drug costs and inadequate healthcare infrastructure.
The International AIDS Society or IAS welcomed the news but cautioned that many countries remain excluded from the initial rollout. IAS President Beatriz Grinsztejn emphasized that “The licensing agreements enabling generic versions of the HIV prevention drug, lenacapavir, in certain countries is an important step forward, but large parts of the world remain excluded, including countries where trials were conducted.”
Grinsztejn expressed optimism about the speed at which these agreements were made but urged for broader access. “We are hopeful that the speed with which these agreements were reached will be maintained, and that the rest of the world will soon benefit from similar agreements to make lenacapavir more affordable and offer a further potent option in the HIV prevention toolbox,” she added.
Gilead Sciences, a US-based biopharmaceutical company, is advancing HIV prevention by partnering with six pharmaceutical manufacturers to produce generic versions of its drug, lenacapavir, for 120 low- and lower-middle-income countries, including Nigeria and Kenya. Lenacapavir, an injection administered twice a year, has shown high efficacy in preventing HIV infection, with clinical trials revealing positive outcomes among at-risk populations in regions such as South Africa, Uganda, Argentina, and the US.
Despite its effectiveness, the drug is currently priced at $42,250 annually in the US, whereas production costs for lower-income areas could be as low as $40 per patient. To address this cost barrier, Gilead is working to offer the drug at reduced prices in high-incidence regions, thereby improving accessibility for vulnerable communities.
Gilead plans to initially prioritize 18 countries that bear 70% of the HIV burden, as identified with external partners. However, the International AIDS Society cautioned that many regions remain excluded from the initial rollout. Despite this, there is optimism for rapid expansion to include more regions, thereby enhancing global HIV prevention efforts.